Imagine a world without disease. Sounds utopian? AI just made it a multibillion-dollar bet to discover drugs – and forget Big Pharma, Big Tech is all in on it, finds Satyen K. Bordoloi.
In February 2020, when the fears of a COVID-19 pandemic loomed, the World Health Organization estimated an 18-month timeline for the first vaccine. Yet, the first mRNA vaccines emerged in half that time—a feat made possible by computational tools and artificial intelligence. That, and AI’s use in even COVID-19 diagnosis, proclaimed one simple truth: AI is here, and maybe AI will cure every disease.
Five years later, that ‘maybe’ has turned to certainty. And that is because AI targets every aspect of novel drug discovery: from the exorbitant costs, decades-long development, testing, and analysis timeline, to a 90% clinical trial failure rate. The goal is not just to accelerate drug development. It is to overhaul everything and remake the foundational process from target identification to clinical trial optimisation.
The ultimate goal, though, like Bryan Johnson, no one involved in it says it: eradicate all diseases. Not like maybe, but literally. Eliminate every disease and keep pipelines ready to attack any new ones that might evolve.
The end result: turning 100 will, in the next couple of decades, become as normal as being 75 is today. And beyond just maintaining health, AI entering medicine is humans entering the realms of reaching ages up to 150. As I don’t tire of repeating, the first human who’ll touch 150 might already have birthed her children.
I segregate this post-AI machine-human collaboration on drug discovery into two main categories. One is the use of AI tools in direct drug discovery. The second exciting field is using generative AI tools as a sparring, research partner for scientists. Both are changing medicine faster than you can say hallelujah.
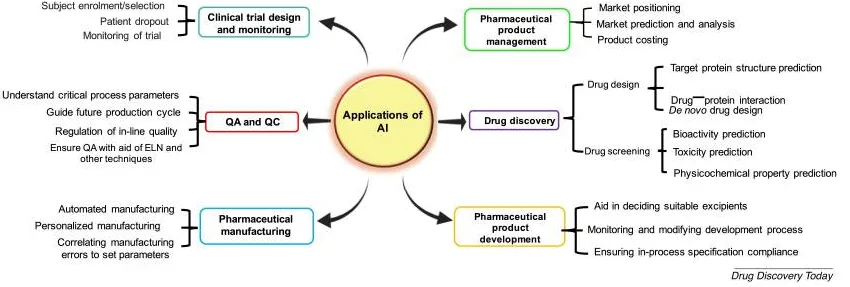
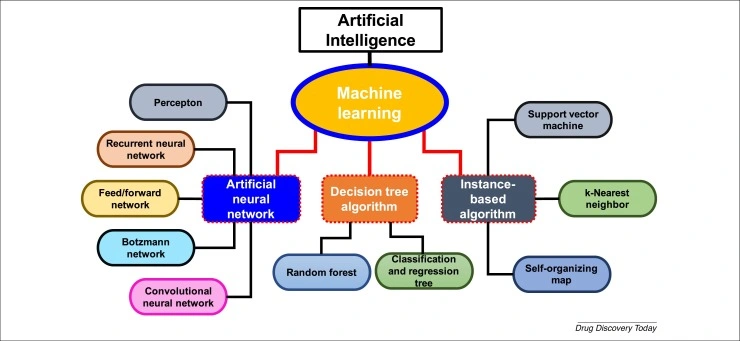
AI, ML, and DL in Drug Discovery
Let’s begin with the fundamentals. The cure to every known disease already exists in the world in the form of chemicals and molecules to treat them. The point is to find which molecule treats which disease. Well, that’s not too difficult, you might think. Yes, till you know the number of drug molecules possible in the world? One 2015 research paper found that this mindboggling number is 10^60. This is so large that you can practically consider it infinite, exceeding even the estimated number of stars in the universe.
Remember those columns you matched as a child: the left is the question, the right is the answer. This is similar. Except in the left column are the 20,000 diseases we know of, and the right column has 10^60 molecules. You know the correct cure for each of the 20,000 diseases is in the right column. However, matching the diseases to their cure could take a really long time.
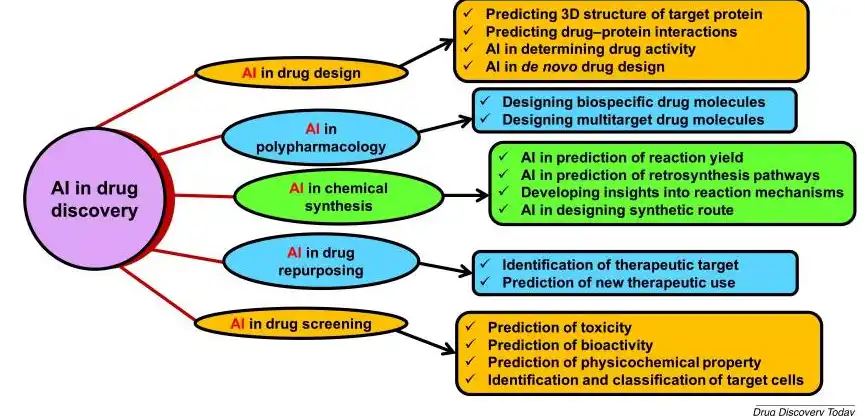
This is where the integration of AI, machine learning (ML), and deep learning (DL) in drug discovery is revolutionising how scientists approach complex biological challenges, including diseases. Traditional methods used trial-and-error experimentation and high-throughput screening that took decades to match just one disease with a cure. As expected, they struggle with the vastness of the chemicals available. AI, however, thrives in such complexity.
Many companies are creating tools to tackle the complexities of drug discovery so humanity can match the two columns more quickly.
Virtual Screening and Molecular Design
Let’s start with the effort to match the disease column correctly with the molecules column. The process of screening billions of compounds for drug-like properties has been streamlined by convolutional neural networks (CNNs) and reinforcement learning. Take Atomwise’s AtomNet. Give it a disease and it will screen over 10 billion molecules in days to match it, achieving a 74% success rate in identifying hits for “undruggable” targets like TYK2, a protein responsible for autoimmune disorders.
Lest we forget, there’s DeepMind’s AlphaFold3, which transformed structural biology by predicting 200 million protein structures and their interactions. It won its founders Nobel Prizes while enabling rapid design of molecules that bind to elusive targets like GPCRs and ion channels.
Then there’s Europe-based Cradle Bio, which uses generative adversarial networks (GANs) trained on a vast library of billions of known working protein sequences to engineer and test new lab proteins based on what scientists want them to do. Partnering with Novo Nordisk and Johnson & Johnson, Cradle accelerates R&D for therapies targeting metabolic disorders and autoimmune diseases.
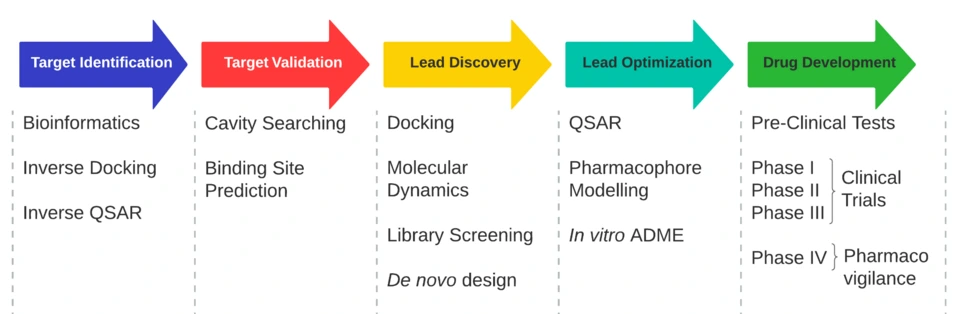
Target Identification and Validation
AI platforms like Insilico Medicine’s PandaOmics analyze multiomics data – genomic, proteomic, and transcriptomic – to pinpoint new therapeutic targets. Take its fibrosis target, where Insilico identified a drug candidate and advanced its AI-designed drug INS018_055 into Phase II trials in under 18 months, a first for the industry.
Similarly, the startup BenevolentAI employs graph neural networks to map disease mechanisms. This has led to collaborations with AstraZeneca and Merck to explore targets for neurodegenerative diseases. These tools uncover hidden patterns in datasets too vast for human analysis, such as protein-protein interactions or synthetic lethality in cancer pathways.
Predictive Modeling for Safety and Efficacy
ML models have become so good over the years that they now manage to predict ADMET (absorption, distribution, metabolism, excretion, toxicity) properties of molecules with previously unheard-of accuracy. Iktos’ Makya, does ADMET properties, 3D Ligand-based & Structure-based modelling, SAR modelling (Structure-Activity Relationship, i.e., investigates the relationship between a molecule’s chemical structure and biological activity.
It also generates optimized small molecules by iterating through that right column of chemicals, claiming that “it explores a huge chemical space (1027) to design novel molecules.” Then there is Gubra, whose StreaMLine refines peptide therapeutics for enhanced receptor selectivity.
These and many other AI, ML, and DL tools mitigate the risks of late-stage clinical failures, which account for nearly 40% of drug development costs. And all with the click of a button and the convenience of a normal computer.

Generative AI: The Collaborative Partner in Innovation
Now, the second, and to me, the really fun, creative force enabling the designing of novel molecules and simulating biological processes, beyond traditional methods, is Generative AI. These tools are similar to the ones I used to research these articles and find these companies from hundreds of them out there (my own AI sorting).
While the other space is mainly occupied with specialist startups, this is the area where the biggies are trying really hard to push the envelope.
Every company worth its AI has tools specifically for research, including drug discovery. Google has Co-Scientist, which has done a stellar job, while Microsoft has tried to match it with its own tool, Discovery. OpenAI has Deep Research, while Claude has a Research agent feature, which they are giving out to scientists under their AI for Science program.
Interactive AI for Hypothesis Generation
Tools like Google DeepMind’s TxGemma, a collection of open-source large language models (LLMs), allow researchers to query AI for insights, such as why a molecule might exhibit toxicity, and through that, generate synthesis routes.
It is also part of Agentic-Tx, an advanced agent AI system built on Gemini that integrates 18 specialised tools and can handle multi-step reasoning using data from PubMed, Wikipedia, and many gene and protein databases.
Synthetic Data and Digital Twins
Companies like UnlearnAI create AI-driven “digital twins” of patients and use them to stimulate clinical trials to reduce control group sizes by 50%. This approach, validated in Alzheimer’s research, slashes trial costs and expedites regulatory approvals. Similarly, Rowan Labs’ Egret-1 leverages decentralised AI to simulate quantum-level chemical reactions, predicting pharmacological properties like protein binding affinity with accuracy surpassing physical experiments. Just chat with it, feed the data, and voila, you have what you need.
Advantages of AI in Drug Discovery
In the overall analysis, the advantages of using AI in drug discovery go beyond financial benefits. After all, what monetary value can you assign to 10 more years not just of life, but an active one? Yet, purely from a development standpoint, the following four benefits can be attributed to the same.
- Cost and Time Reduction: The average cost of developing a new drug is estimated at around $2.23 billion and takes over a decade. AI can slash the timeline to as little as 12 to 18 months and cut at least 40% of the cost. Take the example of Exscientia’s DSP-1181, the first AI-designed drug to enter clinical trials. It reached Phase I trial in 12 months—a fraction of the traditional 4–5 years it takes for a drug of that type.
- Enhanced Success Rates: AI also increases drug Phase I success rates from 40–65% to 80–90% by improving target validation and toxicity prediction. Take the case of BenevolentAI, which used AI to identify baricitinib as a COVID-19 treatment in just two days in February 2020.
- Democratisation of Innovation: Open-source platforms like AlphaFold3 and decentralised AI networks like the partnership of Rowan Labs with Bittensor lower entry barriers, enabling smaller players to compete with Big Pharma. If you have 10^60 to sieve through, you need all hands, big or small, on deck.
- Personalised Medicine: AI integrates genomic, proteomic, and clinical data to tailor therapies. Take TempusAI, which uses ML to match cancer patients with trials based on genetic profiles, doubling enrolment rates.
Despite its promise, AI in drug discovery faces hurdles like biased training data, algorithmic opacity, and regulatory scepticism. In the US, the Food and Drug Administration established the CDER AI Council in 2024 to address these issues through frameworks for ethical AI use and real-world data validation..
From its public recognition during the COVID pandemic to now, where it is enmeshed in every aspect of drug discovery, AI has gone beyond being just an aid to drug discovery. It has become a change agent for the field of medicine. From slashing timelines to unlocking “undruggable” targets, it touches every aspect of medicine. Today, it is not just a means to find treatment for a few diseases; it is the philosopher’s stone to eliminate every one of them.
In case you missed:
- 2025 – Year of Gene Therapy: Curing Lymphoma & Wiskott-Aldrich Syndrome, & Using AI
- From Generics to Genius: The AI Revolution Reshaping Indian Pharma
- The B2B AI Revolution: How Enterprise AI Startups Make Money While Consumer AI Grabs Headlines
- Great quantum poker: Who’s bluffing, and who is holding the aces?
- AI as Cosmic Cartographer: Teen’s Discovery Illuminates Positive Power of Artificial Intelligence
- AI’s Top-Secret Mission: Solving Humanity’s Biggest Problems While We Argue About Apocalypse
- The Great AI Correction of 2026: Why the ‘Bubble’ Popping Could be the Sound of Growing Pains
- How India Can Effectively Fight Trump’s Tariffs With AI
- The Cheaper Than Laptop Robot Revolution: How China’s Unitree Just Redefined Our Future
- How Does AI Think? Or Does It? New Research Finds Shocking Answers