A super artificial intelligence that is as smart as an architect and may predict the molecular configuration of proteins to a great extent.
Try to imagine your body as a large city where there are millions of tiny employees constantly on the move. These employees, called proteins, are of various sizes and shapes and are all monofunctional. But how do these single but indisputably essential proteins design their unique form as if they are made by special origami skills? This over time has become a ‘science fiction conjecture’ that has immensely hit the area of disease diagnosis and drug development.
Now, there’s a game-changer: AlphaFold 3. This is a super artificial intelligence that is as smart as an architect and may predict the molecular configuration of proteins to a great extent. But AlphaFold 3 doesn’t end there. This is like having a universal translator that identifies the shapes of proteins and DNA, RNA, and even possible drugs. This real-time problem-solving tool is all set to redefine medicine, material sciences and our understanding of life.
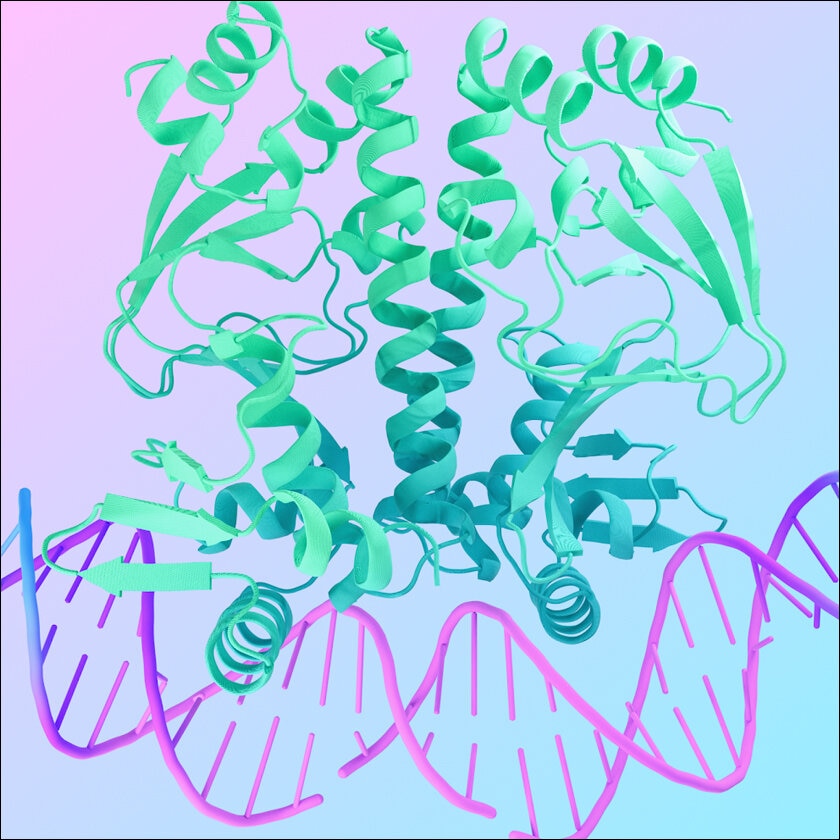
Reasons why understanding protein folding is essential
Protein folding is not a mere twist in the protein; it is an essential step that defines the destiny of a protein in the cell. Suppose a protein is like a complicated mechanical device; the pattern in three dimensions represents how the protein will work. For instance, a wrench would not work properly if it resembles a spoon, and a protein distorted during the folding process cannot achieve the goal it is designed for.
Protein misfolding problems can be severe. Proteins need to be folded properly to be able to do their jobs, so if they are folded incorrectly, they can become dysfunctional. This may result in a condition called protein aggregation, whereby these misfolded proteins form toxic conglomerates in cells within the body. These protein aggregates are toxic to cells and are linked with many incapacitating diseases like Alzheimer’s, Parkinson’s, and cystic fibrosis.
Understanding protein folding is like understanding a hidden programming language within the cell. This allows researchers to understand the behaviour of various proteins, particularly how the molecules interact with others, how they execute their tasks within cells, and how failures in folding lead to diseases. Such knowledge contributes to the testing and creation of novel therapeutic interventions that might help in preventing protein aggregation or in addressing it once it starts.
The Evolution of AlphaFold
AlphaFold was initiated to solve protein folding, a task that had remained unsolved for years. These are macromolecules made up of long chains of amino acids that must fold into these specific three-dimensional structures to perform their functions. Thus, the necessity of protein structure identification rises, as misfolded proteins cause diseases.
AlphaFold was trained using approximately 100,000 protein sequences and their corresponding structures. It was evaluated through the Critical Assessment of Structure Prediction, or CASP, where research groups predict protein structures and then compare them with actual data. As for CASP13 held in 2018, the AlphaFold team took first place and outperformed their competitors. It was not until 2020 that CASP14 saw AlphaFold 2 obtain such high accuracy that many thought the protein folding problem had been solved. Since the announcement of AlphaFold 2, the methods paper detailing the technology has been cited more than 20,000 times, ranking the publication in the top 500 most cited papers in history.
In 2024, DeepMind and Isomorphic Labs unveiled AlphaFold 3, which can foresee the form and behaviour of all elements of life, ranging from DNA to RNA and many more small “drug-like molecules” known as Ligands. This is quite revolutionary, as AlphaFold 3 is no longer limited to proteins but has branched out to DNA and RNA, which are central to gene regulation and, consequently, genetic disease treatments. Moreover, the model’s capacity to estimate the interactions with small molecules, which are often ligands, can greatly enhance the drug development process.
In what ways is it revolutionising industries
AlphaFold 3’s ability to predict molecule structures unlocks possibilities in various fields:
- Medicine: AlphaFold 3 facilitates faster drug discovery since it effectively captures protein folds. It recently supported a study of a protein that is important for a malaria vaccine. In the past, the images were not clear enough and thus it was challenging to identify the most effective target. So through the support of the AlphaFold 3, researchers were able to identify some key components in helping the vaccine to transition from the laboratory to the clinical trials stage.
- Materials Science: AlphaFold 3 predicts how atoms bond together giving the world the potential for designing new forms of matter with certain characteristics. For example, scientists are using AlphaFold 3 to develop new enzymes to decompose plastics better for achieving an effective method to recycle plastic fully.
- Genomics: The ability to predict DNA and RNA structures changes the landscape of genomics. A few weeks ago, AlphaFold 3 presented a precise architecture of a molecular compound in a crop pathogen. This might aid in defining the relationship between the fungus and plant cells, which may usher in higher disease-tolerance crops.
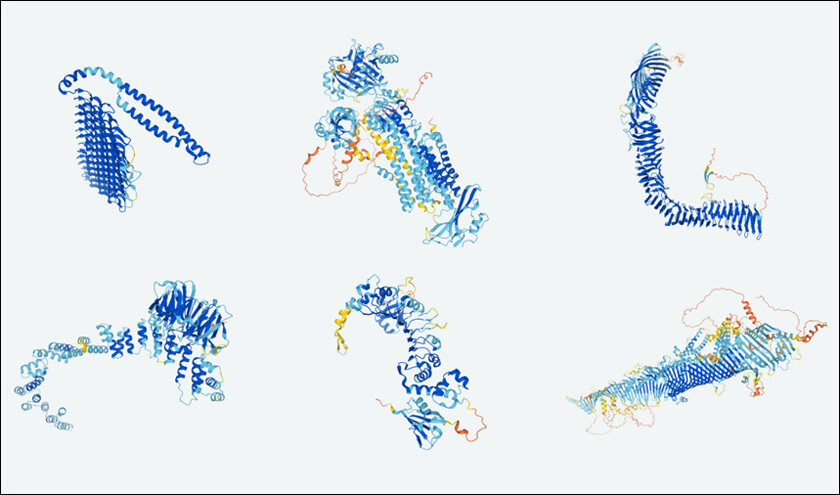
Real-World Applications
- COVID-19 Research:
During the COVID-19 situation, AlphaFold’s technology was instrumental in determining the structure of the SARS-CoV-2 virus. By getting the right structural information on viral proteins, scientists were able to understand how the virus worked and how it reacted with human cells. This was significant to the development of vaccines and treatments to combat the virus and the quick response to the pandemic throughout the world to save millions of lives.
- Rare Diseases:
AlphaFold 3 can help solve the problems posed by rare diseases through the acquisition of additional structural data that may be scarce. For instance, when it comes to cystic fibrosis, a rare genetic disease, the programme can detect the structure of the mutated protein named CFTR. Such structural information allows researchers to develop specific therapies that can rectify or offset the aberrant protein; therefore, enhancing the lives of everyone with such diseases. This approach to therapy holds great potential for several other rare diseases, in which other approaches may prove ineffective.

The Future of AlphaFold
Major progress has been made in molecular biology through AlphaFold 3, but there is still more to be done. Even more fascinating prospects lie ahead for this technology in the future. The use of artificial intelligence and the availability of more data mean that the accuracy of structure prediction from platforms like AlphaFold will only improve over time. Subsequent versions could not only model the future states of static forms but also depict molecular behaviour changes in time, enhancing our understanding of the functions of cells.
Furthermore, it could use AlphaFold to design novel materials with desired characteristics, explain the function and evolution of protein assemblies, and gain a more systems-level understanding of biological systems. A concept of tailored medicine may work because treatments will be based on the specific structures of proteins inherent in a given patient.
Currently, AlphaFold is under development, and its future implementation will create new opportunities for scientific and medical advancement that can transform the existing paradigm and methodology for studying life and diseases. While waiting for such improvements, the effects of AlphaFold 3 will remain ever-present and open new opportunities and possibilities for solutions to some of the most complex issues in the world.









1 Comment
My spouse was diagnosed with Parkinson’s disease. His symptoms included excruciating calf pain, muscular aches, tremors, slurred speech, frequent falls, loss of balance, and trouble standing up from a seated posture. After six months on Senemet, Siferol was given to him in place of the Senemet. It was also at this period that he was diagnosed with dementia. He began seeing hallucinations and became detached from reality. With the doctor’s approval, we stopped giving him Siferol and chose to try the Ability Health Center PD-5 protocol, which we had previously investigated. After three months of therapy, he has made significant progress. The illness has been completely contained. There are no symptoms of persistent twitching, weakness, tremors, hallucinations, or muscle soreness. The PD-5 Protocol was obtained from ability health centre Though you still need to determine what works best for you, I thought I would share my husband’s story in case it could be helpful. Greetings and prayers