While even conservative estimates say it will take over a decade into the future, that’s not a very long time, says Nigel Pereira
In India and around the world, the demand for organ transplants is fast outpacing the availability of human organs from donors. So much so that the health department in Tamil Nadu has put up a stall to encourage organ donors at the 47th India Tourism and Industrial Fair at Island Grounds, Chennai. While the TRANSTAN or Transplant Authority of Tamil Nadu have confirmed that at least 5 people a day have shown their interest in becoming organ donors, it’s still a far cry from how many organs are actually required.
In India alone, a mere 6000 renal transplants occur every year, against a requirement of about 1.8 lakhs. Similarly, a mere 1500 liver transplants occur annually, against a requirement of about 30,000.
Now while there are other options like living organ donors and even animal organ donors, these aren’t really practical solutions. In an interview with CNN health, Dr. Anthony Atala explains that living donors are usually not preferred since in addition to performing surgery on someone who doesn’t need it, you’re also taking away an organ that they do. In the case of animal donors, there are a number of ethical, as well as religious concerns. While they did manage to transplant the heart of a pig into a 57-year-old man with heart failure in 2022, it only added 2 months to his life, making people wonder if the money spent on research could have been better spent on human-relevant treatments.
Bioprinting

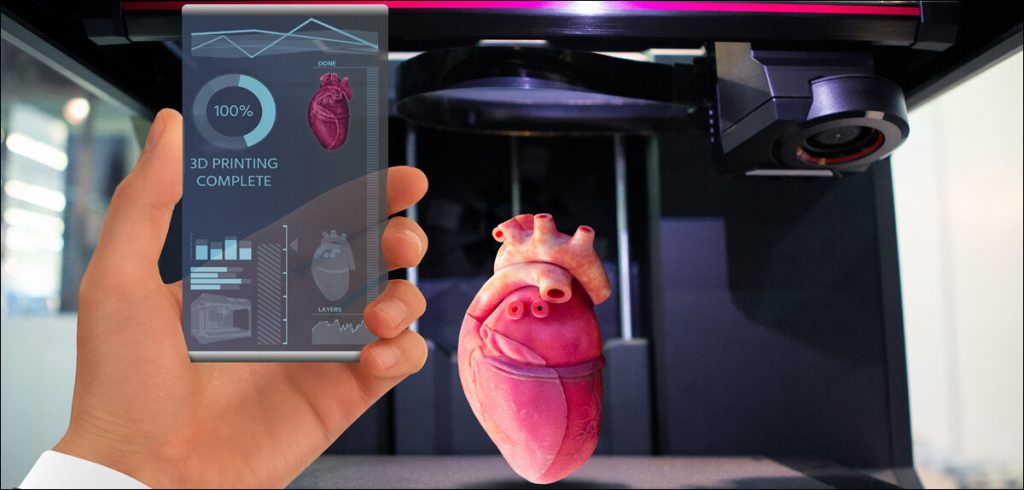
3D medical printing has progressed from dental implants, medical devices, and prosthetics to scaffolds for organs as well as actual human organs. Bioprinting, as the process is called, uses the same principles as commercial 3D printing while changing the material being printed. Instead of using molten plastic that most commercial 3D printers extrude, bioprinters print 3D objects using liquid and gel-based materials, layer by layer.
To print human organs, in particular, a bioink typically made from a mixture of the patient’s own cells and hydrogels which are water-rich molecules that act like glue is loaded into a bioprinter. This bioink, once prepared, consists of living cells, hydrogels, as well as media, and other factors that ensure proper growth and functioning.
Now while this works well for building scaffolds for organs like bladders, livers, kidneys, and even lungs, 3D printing actual organs is a bit harder. This is because of several limitations related to hydrogel-based bioinks, including the failure to support cells that require thick tissue, as well as the absence of cell-sized pores. This is probably the reason why most experts in the field are quite conservative about the timeframe in which fully functional bioprinted organs will actually be available.
While Jennifer Lewis, a professor at Harvard University told CNN health that we are still over a decade away, new inventions and discoveries could shorten that timeframe considerably. The one that looks the most promising is a bioink made with self-generating nanoparticles.
Nanoengineered granular bioink

According to a 2019 study, the success we have had so far in manufacturing scaffolds for organs has relied heavily on jamming a large number of hydrogel-infused particles together to achieve the desired results. The problem, as we have already mentioned in brief, is that this process suffocates the cells due to a lack of microporosity as well as hampers propagation, oxygen transfer, cell penetration, and other growth factors.
As opposed to hydrogels that are bulky, tightly packed, and have an absence of cell-sized pores, microgels programmed with reversible interfacial nanoparticle self-assembly are tiny and loosely packed with well-preserved microporosity. These new “microgels” are referred to as nanoengineered granular bioinks and can be used to construct thick tissue, something that could not be done with conventional hydrogel-based bioinks.
Other problems associated with previously used hydrogels are their inability to retain shape and also their reluctance to integrate with human cells. These problems are referred to as shape fidelity and cell viability respectively. According to Amir Sheikhi, an assistant professor of chemical engineering and biomedical engineering at Penn State, it’s the nanoparticles that help overcome these limitations by enabling the microgels to bond and adhere to each other without being tightly packed. This adhesion mechanism is also reversible which allows the cells to retain shape, grow, and integrate with human cells.
Dr. Amir Sheikhi was quoted stating, “By addressing one of the persistent challenges in the 3D bioprinting of granular hydrogels, our work could open new avenues in tissue engineering and printing functional organs.”
Conclusion
While even conservative estimates of how soon we may have 3D printed organs available to the public are over a decade into the future, that’s not a very long time. Added to the fact that the 3D-printed organ market is estimated to grow to $4.8 billion by 2028, there’s a lot of incentive for science to hurry up with it. To quote United Therapeutics CEO Martine Rothblatt, “I actually believe there is no part of the body that cannot be 3D-printed … including colons and brain tissue.”
In case you missed:
- Tiny robots made from human cells can heal wounds!
- This computer uses human brain cells and runs on Dopamine!
- Researchers develop solar cells to charge phones through their screens
- Age reversal technologies in 2024, longevity escape velocity by 2029?
- Lab-Grown Brain Thinks It’s a Butterfly: Proof We’re in a Simulation?
- Scientists gave a mushroom robotic legs and the results may frighten you
- Scientists have stored the human genome on a Kryptonian crystal
- South Korean firm develops drone that flies on hydrogen fuel
- Mainstream AI workloads too resource-hungry? Try Hala Point, Intel’s largest Neuromorphic computer
- A Glowing Plant Could Be Your New Night Lamp for $29