The first ever gene editing therapies to cure a disease were approved in the UK and US, heralding a new age of medicine writes Satyen K. Bordoloi as he outlines what this means not just for medicine, but life on the planet itself.
The seminal 1997 film Gattaca has one fundamental flaw. In it, embryos are genetically modified to remove diseases, but no genetic treatment seems to exist for human’s post-birth. Surely, if science advanced enough to allow gene edits before birth, we could do that later as well.

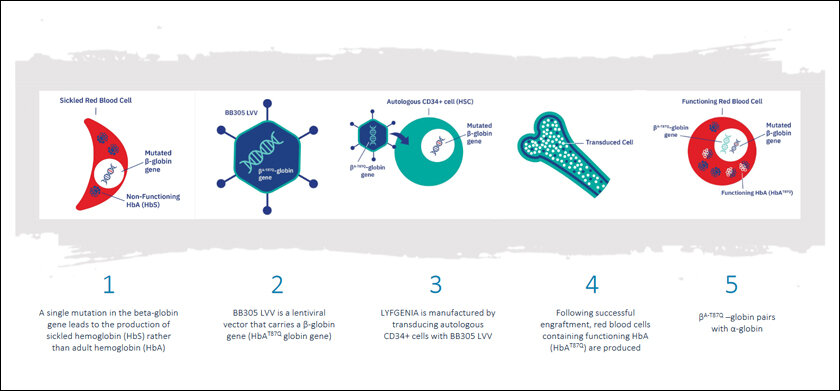
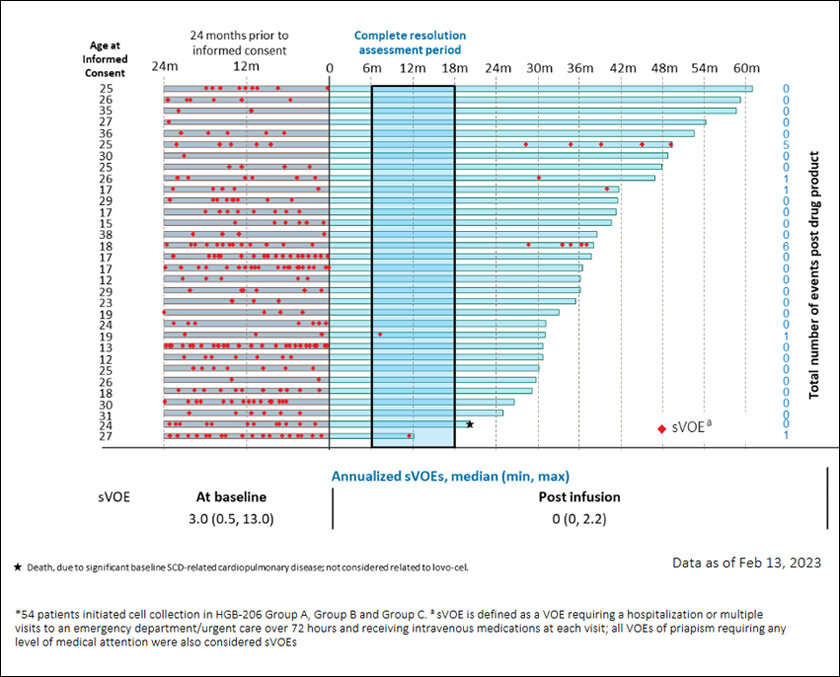
The film’s flaw has been reversed in real life. Last month, the world’s first-ever gene-editing treatment for a disease was approved in the UK. It’s called Casgevy and treats sickle cell disease with the gene-editing tool CRISPR. It was launched by Vertex Pharmaceuticals and CRISPR Therapeutics. Weeks later, the US Food and Drug Administration approved not one but two such treatments for sickle cell, one is Casgevy and the other is called Lyfgenia by Bluebird Bio.
Adjectives can’t describe what this moment means for humanity: ‘dawn of a new era’, ‘breakthrough’ and a ‘game-changer’ pale when we consider what this truly means. The journey to this moment, though, has been full of perils.
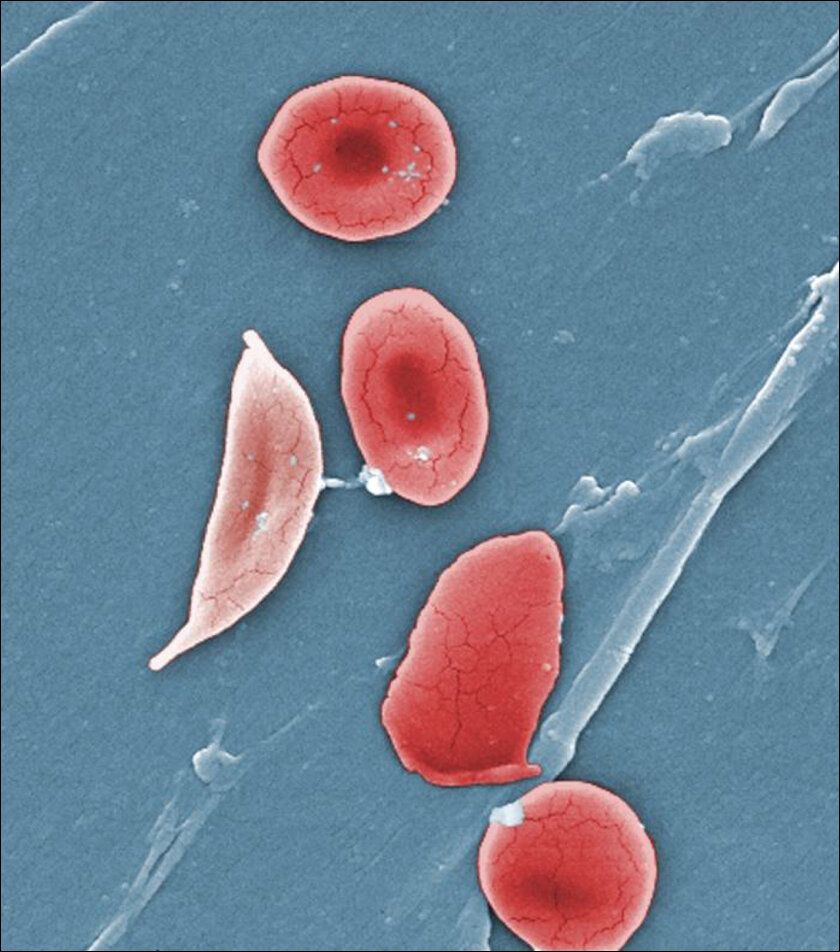
(Image Credit: Wikipedia)
THE JOURNEY TO A GAME-CHANGER:
In 1910, a doctor in the US first saw in his microscope the ‘crescent’ or ‘sickle’ shape of the red blood cells of a man with the disease. This shape was from mutated hemoglobin that made RBCs sticky and reduced their capacity to carry oxygen. In 1948, a Long Island paediatric doctor Janet Watson noticed newborn kids never showed symptoms of sickle cell, even when they developed it later. This was weird because by then it was known as an inborn condition. As this brilliant article in MIT Technology Review highlights, Dr. Watson “hypothesized that the fetal form of the molecule, active in the womb, was protecting babies for a few months after birth, until it was replaced by the adult version: “The theory that at once presents itself is that fetal hemoglobin is unable to produce sickling.””
Though she was right and research on it progressed, it got a boost only after the human genome was sequenced in 2003. By 2007 scientists honed in on the exact variation: BCL11A, which caused the switch from fetal hemoglobin to its adult version. Preventing the switch could cure it. Gene editing CRISPR technology was advancing so the cure, in theory, was simple: mess up BCL11A’s code, thus shutting it, and preventing it from stopping fetal hemoglobin formation.
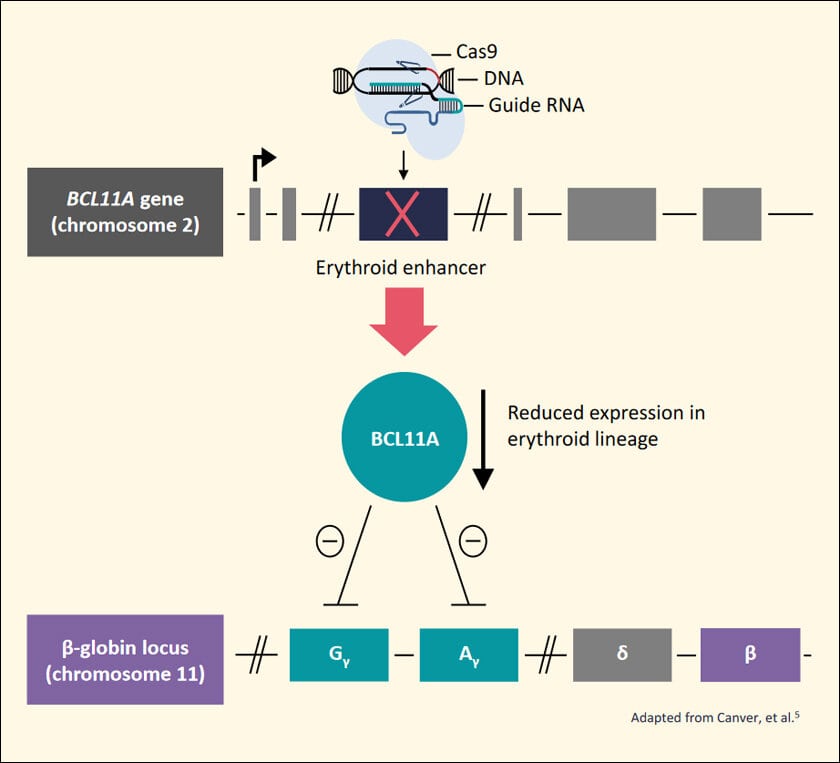
(Image Credit: Vertex Pharmaceuticals)
By 2011, CRISPR was treating sickle cell in mice. But they died in six months. That meant BCL11A was useful for other things we didn’t yet know. Another study found a cluster in a special part of the BCL11A gene active during the production of RBCs. Thus, if they could target only this part, they could leave the rest of the gene intact.
But there was another problem. CRISPR is specific, but when sent to snip something, it will snip that part all over the gene. Thus, the snipping would have to be those DNA bits that do not exist anywhere else to reduce unintended edits. This bit in the cluster was found and nearly a decade of tests and clinical trials later, with over a billion dollars spent only in the Casgevy trial, the miracle cure is ready.
THE AGE OF MAGIC:
While most of us have been busy with the digital world and its advancements, a quiet revolution has been on in something much more important to us: genetics. Like an arrangement of codes running a computer program, DNA is the program that runs life on the planet. It is a set of instructions given to proteins on how to fold, creating everything that is living on the planet. Like the rapidly advancing digital world, the world of life and DNA has seen exponential growth. CRISPR is only one among many emerging gene editing tools, as corresponding discoveries in various fields have supercharged genetics research.
The result is that we are in the age of magic when it comes to meddling with life. We are already bringing dead species to life, not unlike in the film Jurassic Park. We are producing disease-resistant crops and though we can manipulate human life itself, it’s been regulated. Hence, attention has gone on to where gene editing can have the greatest influence: diseases. This cure for sickle cell disease is just the first step into that future.
THE FUTURE:
We are in genetics today where computing was in the 1970s. The major principles of computing had been outlined in the 3–4 decades prior, and computing waited for engineering developments to explode. Fifty years later, the digital world is beyond the imaginings of the founding fathers of computing or any sci-fi writers. No one in the 70s could have imagined that the computing power of supercomputers in their age, almost every household device would carry via the Internet of Things – IoT. Even at the start of the internet age in the 90s, no one could fathom that one day every human would have in their palms, instruments faster and better than their supercomputers.
In the same vein, when it comes to genetics, we can’t even imagine where the world would be in the 2070s. Casgevy seem like magic today, but in 2070, they’d feel about it like we think of transistor radio from a century ago. Like computing, by 2070 everyone would have the power of genetics working for them. We would walk into a neighbourhood genetics lab with our ailments and after analysing our genome, they’d either give us cheaper generic treatments, or a version specialised for our particular need if we could afford it.
Most diseases and disorders today, including life-threatening ones: from retinal blindness and heart diseases to diabetes and many cancers, would be treated at the embryo state like in Gattaca or a little later using treatments like Casgevy. Even conditions like baldness would have a genetic cure.
How will this happen? As our knowledge of genetics increases, we will see patterns. Genetic diseases like sickle cell have shown us that finding the genetic roots of diseases has a lot to do with pattern recognition. As we sequence more people, and put them under special Machine Learning models like those by DeepMind, we’ll discover patterns, i.e. that one gene or sequence found in every human with that disease. We have already found many without using AI. Once found, the cure would be as simple as it is for Sickle cell – you snip that tiny bit of code using something like CRISPR jumbling up that gene and preventing it from giving you that particular disease.
It is a matter of when, not if. If you consider that this first cure took 8 years to clinically trial, the rest could also take time. So instead of 2070, we might have it by 2120s. But like with computing, one success will fuel another and we’ll make exponential strides. This means every new development will go faster than the previous ones.
ARTIFICIAL INTELLIGENCE IN GENETICS:
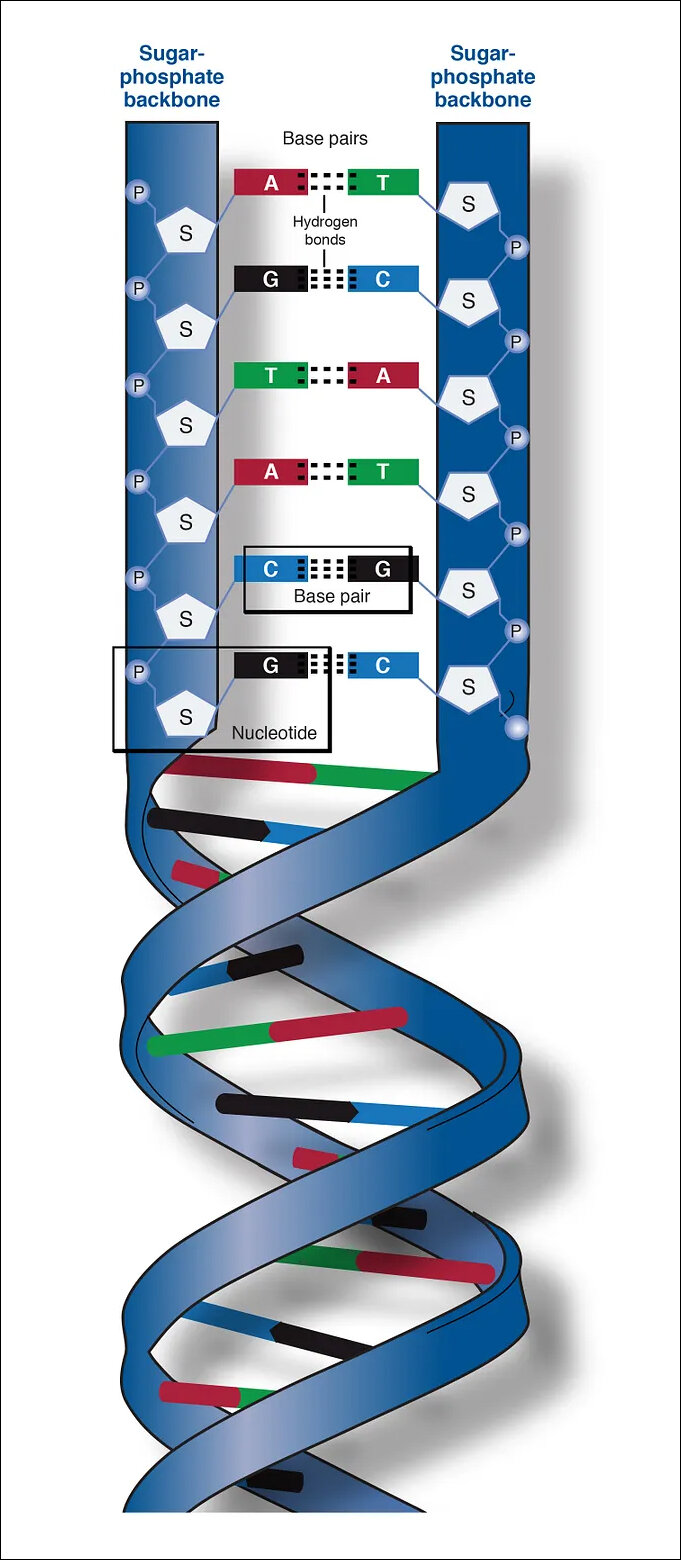
What will hasten this process is, as I wrote before – digital biology. Humans are creatures of symbols. We have turned the world into multiple systems of symbols and sounds we call languages. Playing around with the information stored in these symbols gives us the power to transform the real world. Digital biology is doing the same as biology. Hence, the alphabets A, T, C and G or Adenine, Thymine, Cytosine and Guanine that make up DNA, are not real nucleotides but their representation in human language. We have done the same for everything in the real world, creating a new, fifth dimension of information. As they react in the real world, we use words and systems of symbols to describe and understand them and their interaction. With the advent of the computer, we digitized that which was in black text and images on paper, into digital bits, helping us copy and spread it quicker. This has led to the exponential growth of medicine – along with everything else – in the last half a century.
As we map genomes of more humans and other life on Earth, we’ll use people and Machine Learning algorithms to find patterns. E.g. putting the genome of 10,000 people with schizophrenia into an AI system can help us find the repeat ones, thus helping us find its genetic causes. We can do that for almost any disease and condition.
DOWNSIDE OF THE GENETICS REVOLUTION:
The earth is a laboratory. Life evolved on it via a mix of conditions set in the centrifuge of time. What has taken the longest to evolve and perfect, is life. Every unicellular organism to a huge mammal is here thanks to this genetic churning and evolution going on for at least 3.7 billion years. Humans did not even exist when Dinosaurs roamed the planet. About 3 million years ago, our ancestors started to diverge from our nearest cousins, developing cognition and transforming the planet beyond anything that existed in the planet’s 4.5-billion-year existence. Despite this, till a few decades ago, we did not have the power to touch the very code of life: DNA.
Today, we can alter nature’s 3.7 billion years of work overnight. The consequences could be dangerous. Lyfgenia by Bluebird Bio, has been given a black-box warning by FDA stating that in rare cases, it can cause certain blood cancers. Every genetic medicine we inject is a human-inflicted alteration in the person’s gene. A decade of a clinical trial isn’t enough to understand its unintended effects. Maybe we will truly know the consequences of meddling with DNA a century from now when we discover people treated with these cures pass on the mutation to their children.
Every magician will tell you that the tougher the trick, the greater the danger. This age of magical genetic cures we are entering, better than even imagined in a sci-fi film like Gattaca, likewise comes laden with greater risk. As we bask in the light of its miracles, we must never forget to prepare for any shadows that might lurk under its brilliance.
In case you missed:
- Reversing Heart Disease: Next Step in Living 150 Years Achieved in Lab
- A Howl Heard Worldwide: Scientific Debate Roars Over an Extinct Wolf’s Return
- Prizes for Research Using AI: Blasphemy or Nobel Academy’s Genius
- AI as Cosmic Cartographer: Teen’s Discovery Illuminates Positive Power of Artificial Intelligence
- How Old Are We: Shocking New Finding Upends History of Our Species
- AI Taken for Granted: Has the World Reached the Point of AI Fatigue?
- To Be or Not to Be Polite With AI? Answer: It’s Complicated (& Hilarious)
- AI’s Top-Secret Mission: Solving Humanity’s Biggest Problems While We Argue About Apocalypse
- Why a Quantum Internet Test Under New York Threatens to Change the World
- Google’s Willow Quantum Chip: Separating Reality from Hype