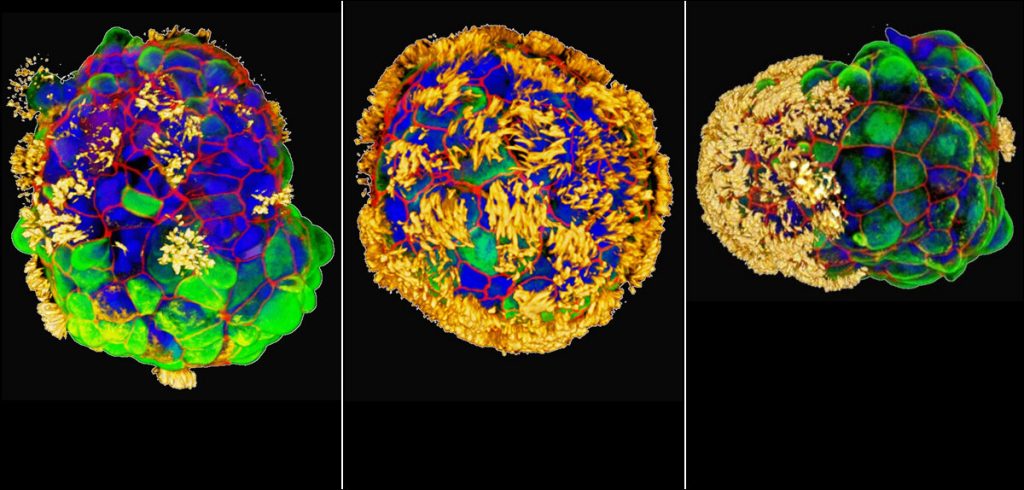
While these “Anthrobots” are about 30 to 500 micrometers in size and are made from cells from the human trachea, the applications could be endless, from neurodegenerative diseases like Parkinson’s or Multiple Sclerosis to irreversible retina damage, and beyond.
To be clear, the robots we are about to talk about in this post are biological and do not contain any mechanical parts, chips, or batteries. That being said, however, they do act a bit like robots, are completely independent, and are made from clumps of biological cells. Why are they called robots then? Well, it all started when scientist Michael Levin claimed he had created “biological robots” from the cells of an African clawed frog and claimed these robots were a new type of organism. He then backed up that claim by showing the world that these Xenobots (named after the frog’s Latin name) could not only spontaneously self-assemble from the frog’s skin but also exhibit “diverse behavior” while swimming through a liquid.
Anthrobots
Now the problem here is that for most biologists whose life work it is to study these cells, this isn’t news of any sort. The cells of amphibians are known to clump together and even regenerate body parts when damaged (like lizards losing their tails). Similarly, when Levin and his team used human lung tissue to make what they are calling Anthrobots in 2024, biologists weren’t surprised since they have been using the same clumps of cells (called organoids) to study lung functions since 2010. The difference here is that while the biologists have been doing everything in their power to immobilize these organoids (or Anthrobots) so that they can be studied, Levin and his team wanted to see what they can do on their own.
According to Levin and his team, these Anthrobots which are about 30 to 500 micrometers in size, are made from cells from the human trachea and can move on their own. In an attempt to describe these tiny robots better, they’re ciliated clumps of cells, meaning they have tiny fibers on them that help them move and they do so by beating these tiny fibers in a single direction. The tiny fibers originate in the human trachea and are what we usually use to move phlegm out from our lungs and into our mouths so we can spit it out. While our tracheas are hollow in the middle with the cilia or fibers pointing inward like early versions of organoids, Anthrobots are spherical with the fibers pointing outward.
So what can they do?
So the question that begs to be asked here, is what can these biological, fiber-covered robots accomplish that makes them worthy of being called a new type of organism? Quite a lot, according to Levin and his team of researchers. To quote him, “What’s never been shown before is the effect these things have on other cells.” He then goes on to explain how the Anthrobots have a healing effect on human tissue that has been damaged. This was demonstrated by letting the Anthrobots loose over a layer of human neurons (in a petri dish) that had been damaged by a scratch. The result was that the Anthrobots encouraged the neurons in the damaged area to grow back without any “mechanical” intervention.
Mechanical intervention here refers to the possibility that this healing effect takes place because the organoids were effectively bridging the gap between the neurons. This alternate theory was dismissed when an inert polysaccharide gel was used instead of the Anthrobots with no healing taking place whatsoever. What this means is that there is some kind of communication taking place between the Anthrobots and the neurons that they are encouraging to heal themselves. Fascinating as that may sound, the team of researchers doesn’t claim to know exactly what’s going on. To quote one of the lead researchers Gizem Gumuskaya, “We don’t know the mechanism, and that’s one of the things we’re trying to figure out,” she then goes on to explain that what they do know about the process is that it’s not merely mechanical.
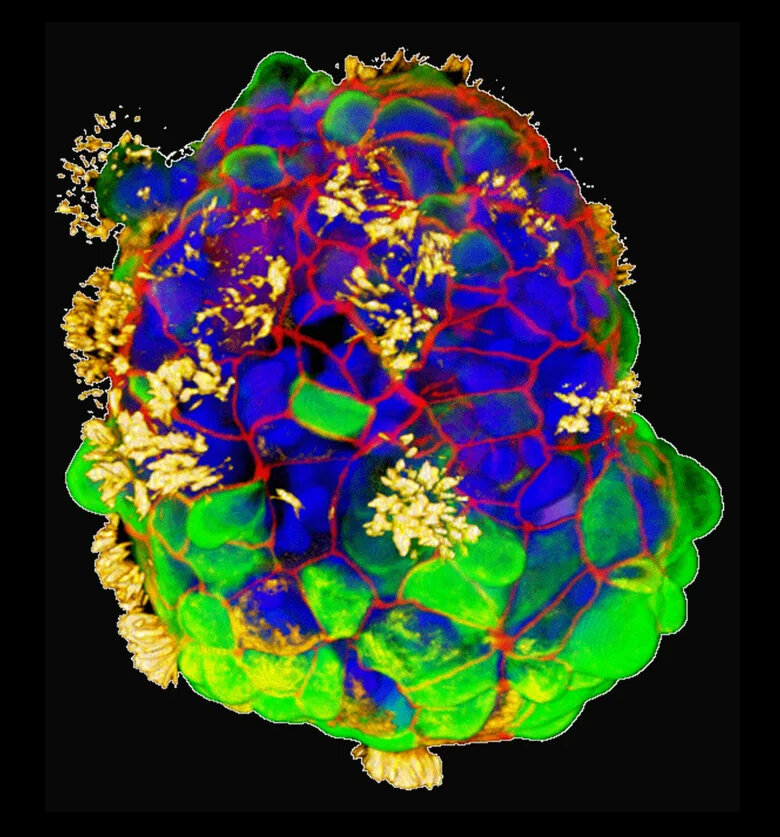
Just the tip of the iceberg
In conclusion, Gizem Gumaskaya believes that what they have achieved so far is just the tip of the proverbial iceberg and she might be right. Call them Anthrobots or Organoids or anything for that matter, if they can encourage damaged cells to heal, that’s a world of possibilities opening up. From everything from neurodegenerative diseases like Parkinson’s or Multiple Sclerosis or even irreversible retina damage, the applications could be endless. And while there are always going to be nay-sayers and people who believe that researching clumps of cells is a giant waste of time, a ray of hope for people with debilitating diseases is always a good thing, even if it’s in the form of ciliated clumps of cells that can swim around a petri-dish.
In case you missed:
- This computer uses human brain cells and runs on Dopamine!
- Lab-Grown Brain Thinks It’s a Butterfly: Proof We’re in a Simulation?
- Scientists gave a mushroom robotic legs and the results may frighten you
- Researchers develop solar cells to charge phones through their screens
- How AI Is Helping Restore the World’s Coral Reefs
- So AI can get bored, “suffer,” and even commit suicide?
- South Korean firm develops drone that flies on hydrogen fuel
- Neuralink Blindsight and Gennaris Bionic eye, the future of ophthalmology?
- CES 2025: NVIDIA’s Cosmos Just Gave Robots a ‘ChatGPT Moment’!
- Slaughterbots: Robot Warriors in the Indian Armed Forces!