As the world faces a power crisis and climate catastrophe, giant batteries have become a necessity, writes Satyen K. Bordoloi as he discovers simple solutions already being rolled out
The 1970s was a crisis decade. Wars in the middle east had disrupted oil supply worldwide. In response, an English chemist – Stanley Whittingham decided to use a 1912 idea to try and make a battery. It would take him and many scientists over a decade to perfect the Lithium-ion battery that would win them a Nobel prize and fuel the 21st century’s great energy addiction by powering its best devices.
Circa 2022. The war in Ukraine has disrupted global oil supply again. Worst still, climate change wreaks havoc worldwide making clean energy a necessity. The planet is desperate for another battery – this time one big enough to power cities.
The problem with electricity is that unlike what we think, nothing is ‘created’. It is just a process where an electron in an atom pushes the next electron all the way from the power plant to your home. Thus, a key solution to global power shortages is to device means to store huge quantities of energy in some other form, as your Li-ion battery does chemically in your gadgets.
What about big Li-ion batteries? Sadly, they are expensive, use rare earth metals which besides being costly are limited and involve environmentally degrading mining, are flammable and worst of all, don’t last long enough to justify cost.

The other reason for needing big batteries is the problems with the way we generate power. Coal is polluting. Chernobyl, Fukushima and now the crisis developing around Zaporizhzhia nuclear power plant in Ukraine prove how dangerous nuclear power can be. The problem with solar: there’s no sun at night; with wind – it’s not always windy.
According to the IEA we will need 10,000 Giga Watts of energy storage by 2040 to meet climate goals that the world has set for itself. This means we have to grow energy storage capacity fifty times in 18 years.
An impossible task? Thankfully, the solutions may be simpler than most of us imagine.
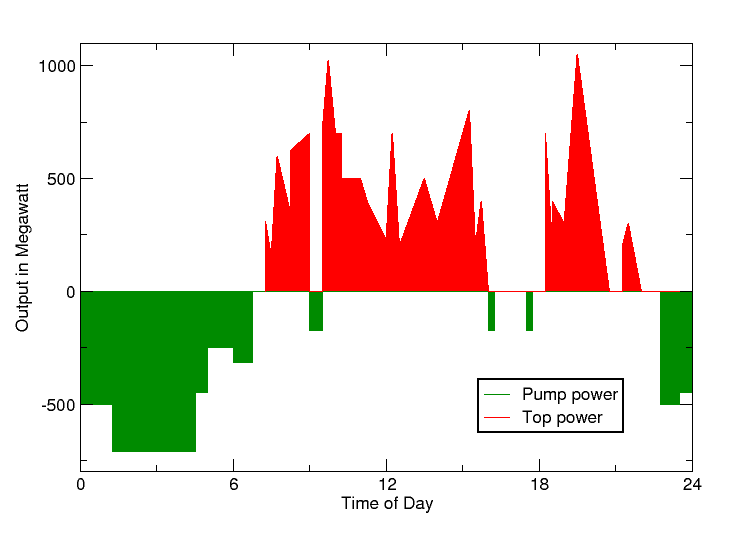
Pumped Hydro-electric Storage: Pumped hydroelectric storage works by moving water between two reservoirs at different heights. When electricity demand is low in the nearby town or city, the facility uses it to pull water from the lower to the top reservoir. The moment there’s a need for electricity, the turbine gates are opened and the flowing water generates electricity just like a hydropower station.
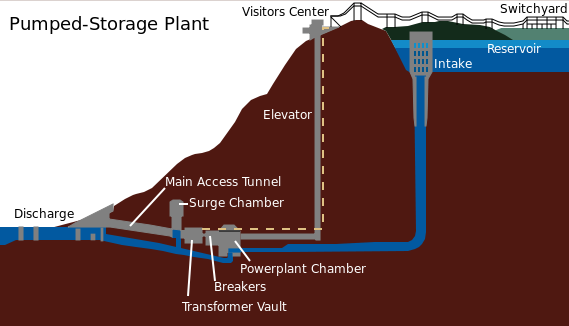
This is invaluable in filling gaps in solar and wind power. Excess power created by solar energy during the day and windmills during high winds can be used to draw the water into the upper reservoir. At night and during times of low wind, the water can be released back where a turbine converts it back to electricity.
Sand Batteries: During winters, most of the electricity in colder nations of Europe is used for heating. Researchers in Finland have come up with the simplest solution to heat houses: sand batteries. The device is nothing but a giant, insulated tank that stores low-grade sand, which during high-energy summer days is charged by heating it using electricity from cheap and clean solar or wind energy. The insulated tank stores the heat at around 500 degrees Celsius for months which can be used to warm homes by cooling the sand chamber when needed during the winters.

This battery has jumped out of the drawing board and was installed in the town of Kankaanpää, 230 kilometres north-west of Helsinki, where homes, offices and even the public swimming pool are being heated by thermal energy stored in its 7-metre insulated container filled with 100 tons of discarded sand from construction.
Iron-Air Batteries: Iron is one of the cheapest and most abundant metals on the planet with one major drawback: it reacts with oxygen and rusts. Interestingly, when taking an oxygen atom while rusting, iron gives off an electron that can be converted into electricity. Researchers have figured out they can use this to generate electricity.

Iron pellets stored in a chamber interact with abundant oxygen in ambient air to oxidize in the same way it does during normal corrosion. This gives off an electron that is converted into electricity. To store electricity or charge the battery, you simply convert the rust back into iron by passing electricity using iron electrodes, generating oxygen in the process.
This simple process does not need special cells to store it, is risk proof and has the potential of grid-scale energy storage making iron-air rechargeable batteries an attractive technology.
Sodium-Ion Batteries: These use a similar idea to Li-ion. The differences are: sodium is 1000 times more abundant than lithium, is up to 40% cheaper, is not as flammable, and has lower energy density so batteries will be heavier. Thus Na-ion batteries may not be useful to run your car but can be great for city-scale grids.
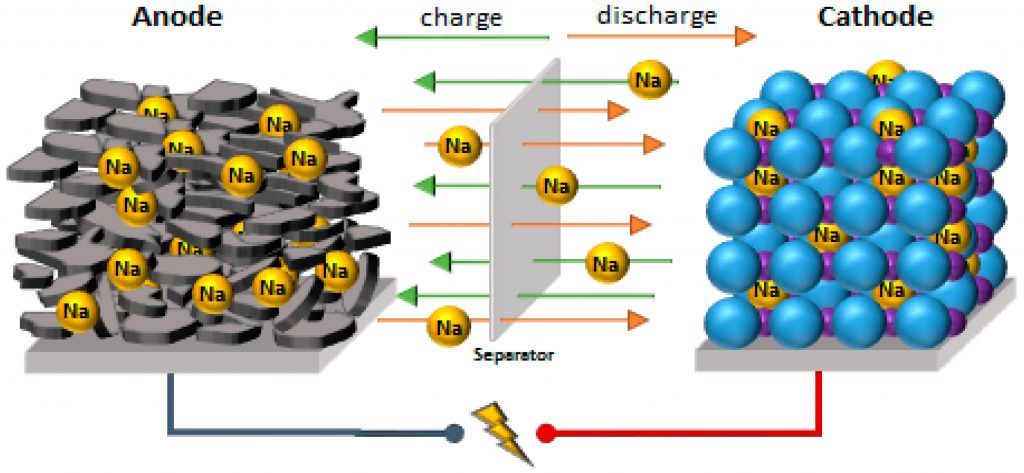
Research is also going on to use calcium, magnesium or zinc in place of Lithium.
Other Solutions: There are a ton of other solutions being researched. One concept hopes to store energy in molten salt i.e., salt is heated thus liquifying it and is used as a heat transfer fluid for thermal energy storage.
The oldest – invented in 1859 – and still widely prevalent rechargeable battery is the lead-acid battery we still use in almost every internal combustion engine because it is cheap and easy to manufacture. It uses an electrolyte with one or more dissolved electroactive elements that flow through electrochemical cells converting chemical energy into electrical energy. The reaction is reversible, allowing the battery to be charged and discharged. Larger versions of it are ubiquitous backup power sources in modern life. However, this technology is also undergoing a revamp.
Hybrid flow batteries instead use one or more electroactive components deposited as a solid layer. The cell has one battery electrode and one fuel cell electrode and uses zinc-bromide, zinc-cerium, soluble lead acid and iron salt.
It is said that ‘necessity is the mother of all invention’. The 1970s oil shortage gave us a battery that powers a large part of the world today. The current oil crisis will also, hopefully, leave us with new grid-scale batteries. This time there’s an added incentive: climate change makes it a such a big necessity that, in a way, the fate of the world, and the human race, depends on it.
In case you missed:
- Would an Electric Plane Have Reduced the Air India Crash Death Toll?
- Nuclear Power: Tech Giants’ Desperate Gamble for AI
- AI as Cosmic Cartographer: Teen’s Discovery Illuminates Positive Power of Artificial Intelligence
- And Then There Were None: The Case of Vanishing Mobile SD Card Slots
- AI’s Top-Secret Mission: Solving Humanity’s Biggest Problems While We Argue About Apocalypse
- A Data Centre on the Moon – From Sci-Fi to Necessity
- Meet Manus AI: Your New Digital Butler (don’t ask it to make coffee yet)
- Rise of the Robolympics: When R2-D2 Meets Rocky Balboa
- Digital Siachen: How India’s Cyber Warriors Thwarted Pakistan’s 1.5 Million Cyber Onslaught
- Project Stargate: Dubious Origins in the 1970s to AI Goldrush in 2025