2025 would also go down in history as the year when gene therapies began to go mainstream, with multiple of them being approved, writes Satyen K. Bordoloi
My first memory of cancer is the younger brother of a classmate. Blood cancer, we whispered between classes in the late 80s. When he eventually succumbed, I remember the pall of grief that spread over, not just my class, but the whole school. Back then, death, for us kids, was something that happened to the very old. But this, to a schoolmate not even ten, and from something we didn’t understand, didn’t make sense. That would be the first time I remember being stripped of my innocence.
Hence, when I heard in December 2025 of two landmark approvals for gene therapies – one for a form of leukaemia and another for the rare Wiskott-Aldrich syndrome (WAS) – it somehow felt personal, like another teen like me somewhere in the world would not have an early personal connect with these deadly diseases thanks to CRISPR. Overall, these successes portend a revolution driven by the accelerating convergence of gene-editing tools and artificial intelligence, promising to redefine treatment for countless other diseases. A world without diseases – once a sci-fi fantasy, now seems possible.
A Living Cure for Lymphoma
The treatment known as Yescarta (axicabtagene ciloleucel) is not a traditional drug but a personalised living therapy. It belongs to a groundbreaking class of medicines called CAR T-cell therapy. For patients with certain aggressive forms of large B-cell lymphoma who have not responded to initial treatments, this therapy offers a powerful new option.
Under the process, a patient’s own T-cells, a type of immune cell, are collected and genetically reprogrammed in a laboratory. Scientists use a deactivated virus as a “vector” to insert a new gene into these cells. This gene instructs the T-cells to produce special proteins on their surface called chimeric antigen receptors (CARs). These are engineered to recognise and latch onto a specific protein (CD19) found on the patient’s cancerous B-cells.
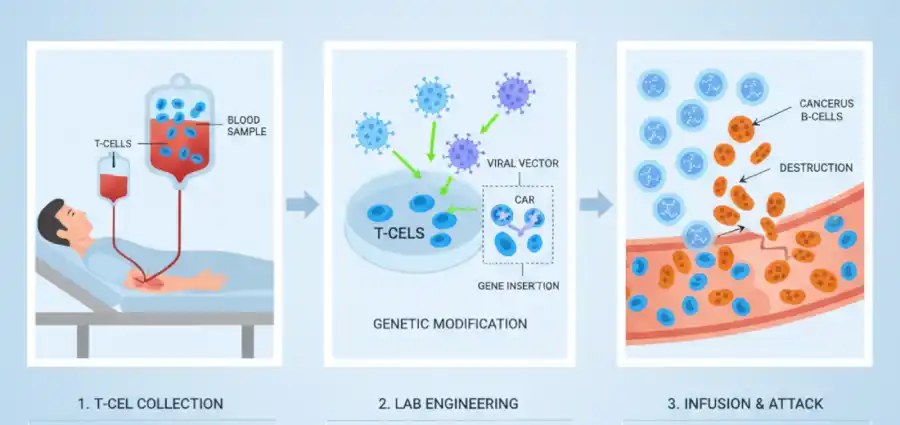
These “hunter-killer” cells are then multiplied and returned to the patient. Here, they patrol the body seeking and destroying cancer cells. The results from a large-scale study have been truly dramatic. Research has shown that some of the very first patients treated with CAR-T therapy over a decade ago remain in remission, suggesting the potential for a durable, functional cure for one of the deadliest killers on the planet.
Wiskott-Aldrich syndrome (WAS) is a rare inherited immune disorder that primarily affects males. It is caused by mutations in the WAS gene and follows an X-linked recessive pattern. The condition is characterised by a clinical triad of eczema, thrombocytopenia (low platelet count leading to bleeding issues), and recurrent infections due to immune deficiency. Symptoms often appear in infancy and can include bloody diarrhoea, frequent infections, and skin problems. Because of its impact on both blood clotting and immune function, WAS can be life-threatening without treatment, and management may involve supportive care, immunoglobulin therapy, or bone marrow transplantation.
A Genetic Reset for Wiskott-Aldrich Syndrome
Just days after the spotlight on cancer, the U.S. FDA approved etuvetidigene autotemcel (etu-cel), the first cell-based gene therapy for Wiskott-Aldrich syndrome (WAS). Now WAS is a rare, life-threatening genetic disorder that mainly affects young boys by causing a mutation in the WAS gene to cause a dangerous triad of immune dysfunction, severe eczema, and an inability to form proper platelets. This can cause bloody diarrhoea, frequent infections and uncontrolled bleeding leading to life-threatening conditions. Treatment so far has involved supportive care, immunoglobulin therapy, and a risky bone marrow transplantation. The problem with bone marrow transplant: a matched donor is not always available.
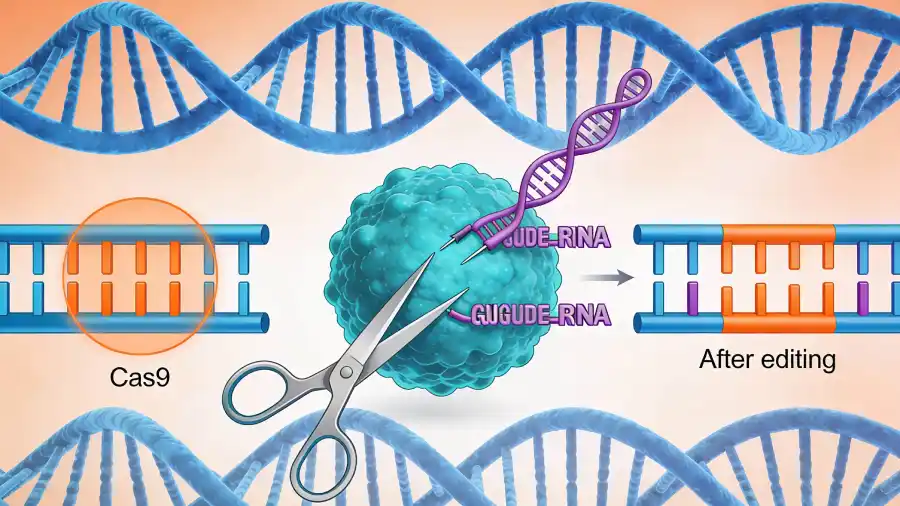
Now, etu-cel offers a truly transformative alternative to these treatment options. The therapy employs an ex vivo (outside-the-body) approach, in which hematopoietic stem cells are harvested from the patient’s own bone marrow. In the lab, a functional copy of the WAS gene is delivered into these cells using a viral vector. After the patient undergoes mild chemotherapy to clear space in their bone marrow, the genetically corrected stem cells are reinfused. These cells then engraft back and begin producing a lifetime supply of healthy immune cells and platelets.
Clinical trial data for this have been compelling. Following treatment, the rate of severe infections plummeted, and the rate of moderate-to-severe bleeding events decreased significantly. With a reported 96% overall survival rate and sustained engraftment of corrected cells over years, etu-cel treatment goes beyond managing symptoms to directly address the genetic root cause of the disease and eliminate it.
The Daunting Challenge of Access and Cost
Both treatments, like other extraordinary CRISPR treatments in clinical trials, are marred by a single problem: their staggering costs and the complexity of access. CAR-T therapies like Yescarta are famously expensive, often priced between $350,000 and $500,000 for a single treatment course. The manufacturing process is highly complex and personalised, making scalability difficult at least right now.
Now CRISPR treatment for ultra-rare diseases like WAS is even more perilous. The small patient population makes traditional drug development financially unsustainable for most pharma companies. And if you analyse the landscape of CRISPR therapy treatments, market forces are pushing companies to focus on diseases with a more straightforward path to profitability, i.e., those with more patients, potentially leaving other rare conditions far behind. This, naturally, creates an ethical and economic dilemma: how do we deliver million-dollar cures in a sustainable healthcare system?
What is urgently needed are innovative payment models, outcomes-based agreements, and increased public funding for foundational research, the last of which is sadly reducing as governments across the world push for austerity. The goal should change from “CRISPR for one” to “CRISPR for everyone“.
The Confluence of Gene Therapy and AI
This is where Artificial Intelligence and everything that comes under this umbrella term – Machine Learning, Neural Networks, Deep Learning, expert systems, NLP, computer vision, etc., is paving the way for a world of medicine and cures unlike any that we have ever fantasised about. AI isn’t just a supporting tool, but a foundational technology to accelerate, refine and redefine every step of the gene therapy pipeline.
Take drug discovery. Traditional drug discovery has been like “tasting hundreds of prepared dishes to find the one that tastes perfect.” However, new AI models, such as PDGrapher, are changing this. By mapping the complex network of interactions between genes and proteins in diseased cells digitally, these tools can predict the precise genetic “pressure points” that will reverse the state of a disease. This allows scientists to identify optimal drug targets or gene editing strategies with unprecedented speed and accuracy, and much before going to clinical trials, saving years, sometimes even decades, in the drug discovery pipeline.
AI is also democratizing gene editing. With so many gene pairs, each linked to another in unique ways and manifesting in still-divergent ways, it takes months of trial and error to find one’s way through the complexity of designing a CRISPR gene-editing experiment. However, AI agents like CRISPR-GPT are flattening this steep production curve by acting as intelligent copilots to guide researchers, and even novices, through experimental design, to predict potential off-target effects, and also troubleshoot problems that might emerge. This has the potential to democratize gene editing, accelerating research in academic and startup labs worldwide.

As highlighted by the World Economic Forum, AI is enabling a shift from reactive to predictive and preventative medicine. The combination of AI with gene therapy is leading us to a future where we can model “virtual patients” to simulate the outcome of a genetic intervention before it is ever tried in an actual living person, leading us to design truly personalised therapies that are based on an individual’s unique genomic and cellular profile.
The approvals of Yescarta and Etu-cel are just the beginning of a new era in medicine, as they prove that it is possible to hack the biological code of devastating diseases and deliver lasting cures. The following two decades will be crucial, defined by the merging of biology and computation, in which AI will give a more sophisticated understanding of life’s language, making gene editing more precise and deliverable.
In this miraculous medical future, the challenge will shift from the scientific to the societal: how do we steer these two technologies – gene editing and AI – towards an equitable access, ethical application, and a future where the most potent medicines are not scarce commodities, but accessible solutions for the healing of every human.
Whatever else happens, one thing is for sure: some teenager somewhere will not have their innocence stripped away because a classmate’s brother died of leukaemia.
In case you missed:
- Cure Every Disease: How AI is Rewriting the Future of Medicine, and Humanity
- A Howl Heard Worldwide: Scientific Debate Roars Over an Extinct Wolf’s Return
- From Generics to Genius: The AI Revolution Reshaping Indian Pharma
- AI’s Top-Secret Mission: Solving Humanity’s Biggest Problems While We Argue About Apocalypse
- AI as Cosmic Cartographer: Teen’s Discovery Illuminates Positive Power of Artificial Intelligence
- Great quantum poker: Who’s bluffing, and who is holding the aces?
- Face-Off: Denmark’s Copyright Move Against Deepfakes & the Ghost of Anil Kapoor
- 100% Success Rate: Johns Hopkins AI Surgeon Does What Humans Can’t Guarantee
- Quantum Internet Is Closer Than You Think, & It’s Using the Same Cables as Netflix
- AI vs AI: New Cybersecurity Battlefield Where No Humans Are in the Loop









